Do you look for 'how to write molecular equations'? Here you can find the questions and answers on the subject.
Table of contents
- How to write molecular equations in 2021
- Total ionic equation
- Example of a molecular equation
- Molecular ionic, and net ionic equations
- Total molecular equation
- Complete molecular equation
- How to write ionic equations
- Molecular equation to ionic equation
How to write molecular equations in 2021
 This picture demonstrates how to write molecular equations.
This picture demonstrates how to write molecular equations.
Total ionic equation
 This picture demonstrates Total ionic equation.
This picture demonstrates Total ionic equation.
Example of a molecular equation
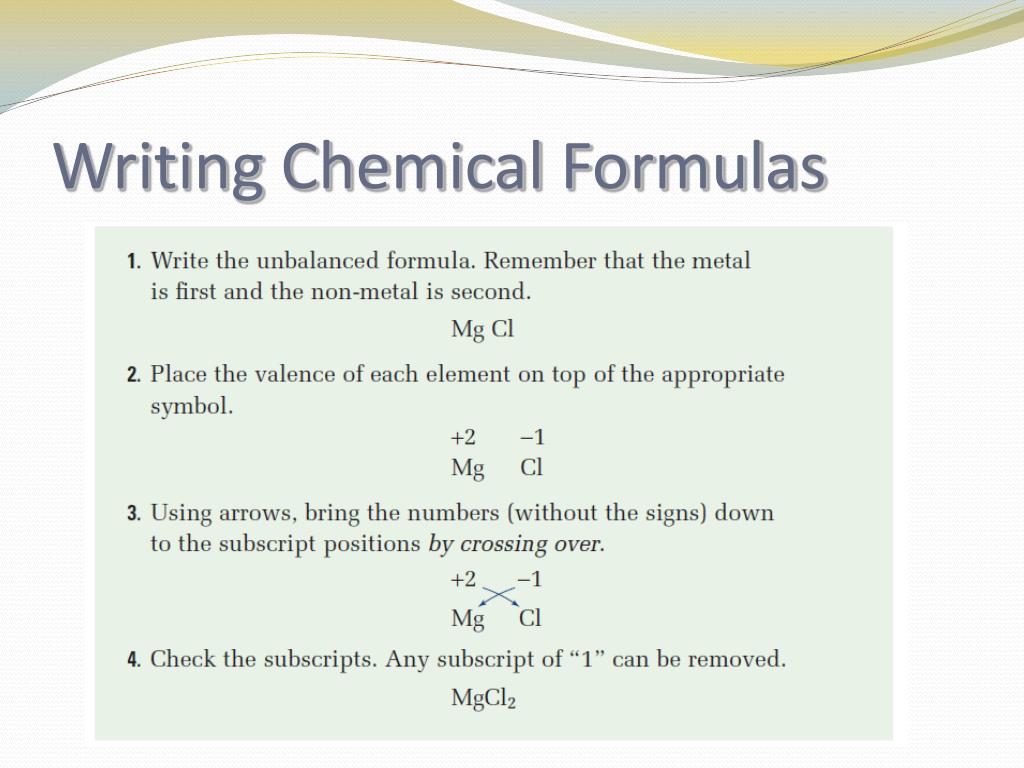 This picture representes Example of a molecular equation.
This picture representes Example of a molecular equation.
Molecular ionic, and net ionic equations
 This picture shows Molecular ionic, and net ionic equations.
This picture shows Molecular ionic, and net ionic equations.
Total molecular equation
 This picture demonstrates Total molecular equation.
This picture demonstrates Total molecular equation.
Complete molecular equation
 This image demonstrates Complete molecular equation.
This image demonstrates Complete molecular equation.
How to write ionic equations
 This picture illustrates How to write ionic equations.
This picture illustrates How to write ionic equations.
Molecular equation to ionic equation
 This image shows Molecular equation to ionic equation.
This image shows Molecular equation to ionic equation.
What is the best way to write the molecular formula of a molecule?
How to Write the Molecular Formula of a Molecule. Step 1: Write a list of the elements present. Step 2: To the right of each element, write how many atoms of this element are present are in the molecule. Step 5: Rewrite the list on one line as a molecular formula.
What is the formula to convert between empirical and molecular formulas?
To convert between empirical and molecular formulas, the empirical formula can be multiplied by a whole number to reach the molecular formula. In this case, the empirical formula would be multiplied by 6 to get to the molecular formula.
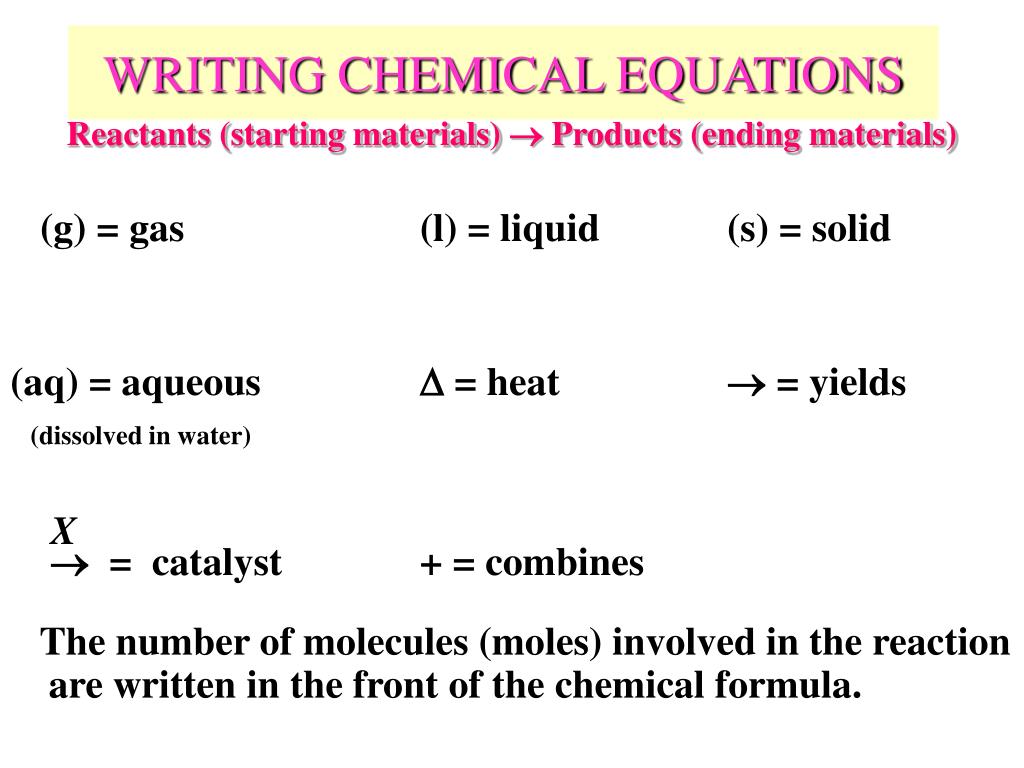
What is the difference between a molecular equation and a chemical equation?
Typically you will be asked to further dissect a chemical equation by writing not only the molecular equation, but additionally the complete ionic and net ionic equations. The molecular equation is the full balance chemical equation.
What is the best way to write a chemical equation for an element?
If you want to write a chemical equation, start by writing the chemical formulas of each reactant. Use the prefixes, such as mono-, di-, tri-, and tetra-, to figure out the number of atoms present for each element, and write this number as a subscript for each element. For example, dihydrogen monoxide would be more easily written as H2O.
Last Update: Oct 2021