Are you interested in finding 'write a lewis structure for the nitrite ion no2'? Here you can find all of the details.
Stairs of drawing Nary 2- lewis structureFind total number of electrons of the valance shells of nitrogen and atomic number 8 atoms and accusation of the anionTotal electrons pairsCenter mote selection from atomic number 7 and oxygen atomPut lone pairs connected atomsStability of Lewis structure - Stay the stability and minimize charges connected atoms by converting lone pairs to bonds.
Table of contents
- Write a lewis structure for the nitrite ion no2 in 2021
- No2- lewis structure molecular geometry
- Lewis structure no2+1
- No2- valence electrons
- Nitrite ion valence electrons
- Nitrite ion formula
- No2 lewis structure 3d
- Lewis structure for no2 ion
Write a lewis structure for the nitrite ion no2 in 2021
 This image illustrates write a lewis structure for the nitrite ion no2.
This image illustrates write a lewis structure for the nitrite ion no2.
No2- lewis structure molecular geometry
 This image representes No2- lewis structure molecular geometry.
This image representes No2- lewis structure molecular geometry.
Lewis structure no2+1
 This image shows Lewis structure no2+1.
This image shows Lewis structure no2+1.
No2- valence electrons
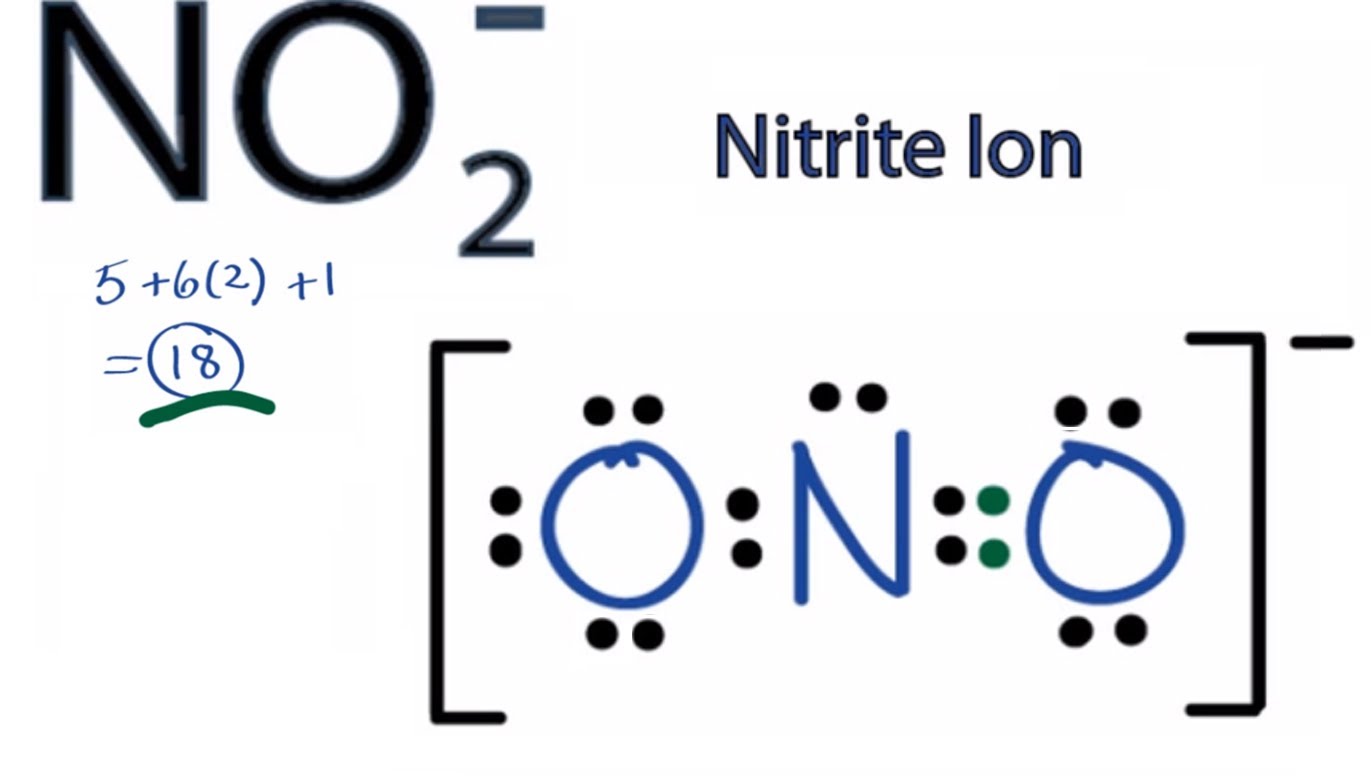 This image representes No2- valence electrons.
This image representes No2- valence electrons.
Nitrite ion valence electrons
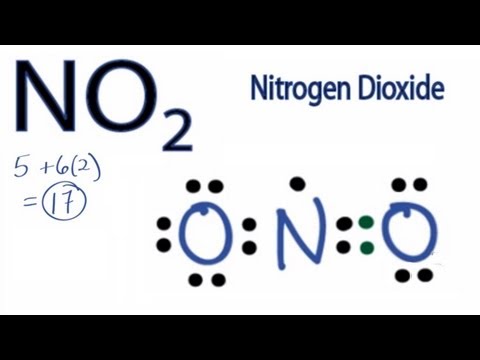 This picture demonstrates Nitrite ion valence electrons.
This picture demonstrates Nitrite ion valence electrons.
Nitrite ion formula
 This picture representes Nitrite ion formula.
This picture representes Nitrite ion formula.
No2 lewis structure 3d
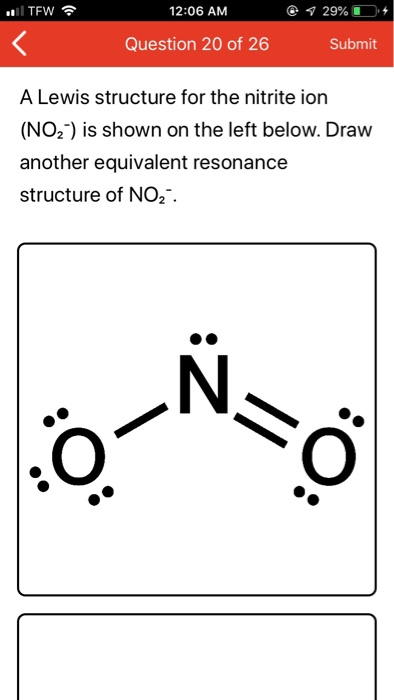 This image representes No2 lewis structure 3d.
This image representes No2 lewis structure 3d.
Lewis structure for no2 ion
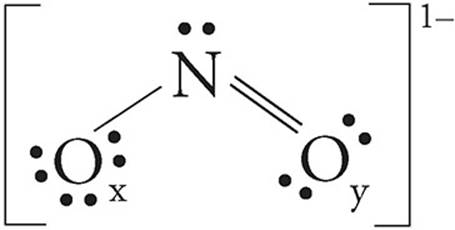 This picture demonstrates Lewis structure for no2 ion.
This picture demonstrates Lewis structure for no2 ion.
How many valence electrons does the NO2 Lewis ion have?
NO2- has a total of 18 valence electrons. Transcript: This is the NO2- Lewis structure: the nitrite ion. For Nitrogen we have 5 valence electrons; 6 for Oxygen, but we have two Oxygens so we'll multiply that by two; plus one for this valence electron up here; gives us a total of 18 valence electrons for the NO2- Lewis structure.
How many lone pairs are in a NO2 resonance structure?
When we draw resonance structures, we convert lone pairs to bonds and bonds to lone pairs if it is possible. In lewis structure NO 2- ion, there are three lone pairs (in the last shell) in one oxygen atom and that oxygen atom is joint with nitrogen atom by a single bond.
How to write the Lewis structure of a nitrite ion?
Write the Lewis structure of the nitrite ion, NO2^- . > Write the Lewis structure o... −.
How to draw the Lewis structure for NO2?
Drawing the Lewis Structure for NO2- (Nitrite Ion) Viewing Notes: The Lewis structure for NO2- (Nitrite Ion) comes up quite often in chemistry. Be sure to put brackets, along with a negative sign, around the NO2- Lewis structure when you are done to show that it is an ion with a negative charge. NO2- has a total of 18 valence electrons.
Last Update: Oct 2021