Do you seek for 'write a nuclear equation for the alpha decay of'? You will find all the information on this section.
Exploratory Decay Equation Fashionable α-decay, the collective number of the product nucleus (daughter nucleus) is cardinal less than that of the decaying nucleus (parent nucleus), while the Thermonuclear weaponA nuclear arm is an detonative device that derives its destructive effect from nuclear reactions, either fission (fission bomb) or A combination of nuclear fission and fusion (thermonuclear weapon). Both reactions release vast quantities of energy from relatively small amounts of matter. routine decreases by 2 In general, the alpha decay equivalence is represented equally follows: A ZX →A−4 Z−2 Y+4 2 He Z A X → Z − 2 A − 4 Y + 2 4 He
Table of contents
- Write a nuclear equation for the alpha decay of in 2021
- How to write nuclear equations for gamma decay
- Alpha particle equation
- Nuclear equation for alpha decay calculator
- Nuclear equation for beta decay
- Alpha emission equation
- Write a nuclear equation for the alpha decay of 23892u.
- How to write alpha decay equations
Write a nuclear equation for the alpha decay of in 2021
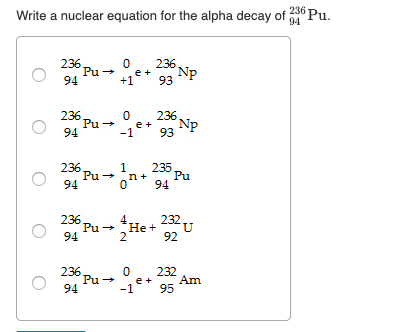 This image representes write a nuclear equation for the alpha decay of.
This image representes write a nuclear equation for the alpha decay of.
How to write nuclear equations for gamma decay
 This image illustrates How to write nuclear equations for gamma decay.
This image illustrates How to write nuclear equations for gamma decay.
Alpha particle equation
 This image demonstrates Alpha particle equation.
This image demonstrates Alpha particle equation.
Nuclear equation for alpha decay calculator
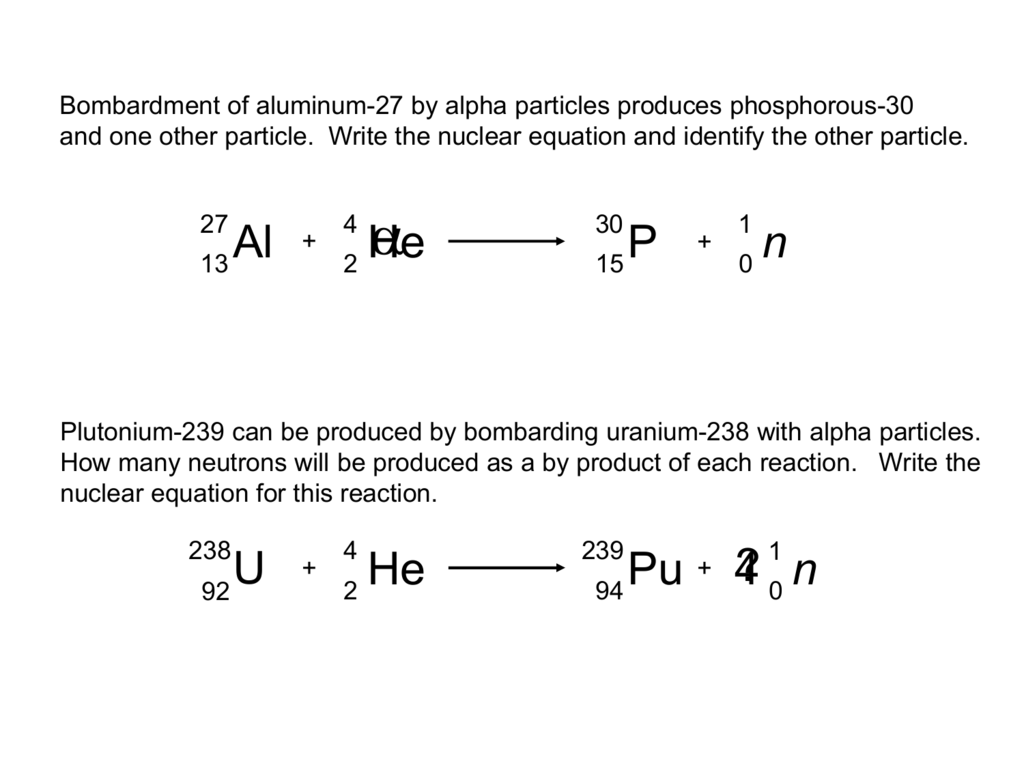 This picture demonstrates Nuclear equation for alpha decay calculator.
This picture demonstrates Nuclear equation for alpha decay calculator.
Nuclear equation for beta decay
 This image demonstrates Nuclear equation for beta decay.
This image demonstrates Nuclear equation for beta decay.
Alpha emission equation
 This image representes Alpha emission equation.
This image representes Alpha emission equation.
Write a nuclear equation for the alpha decay of 23892u.
 This image shows Write a nuclear equation for the alpha decay of 23892u..
This image shows Write a nuclear equation for the alpha decay of 23892u..
How to write alpha decay equations
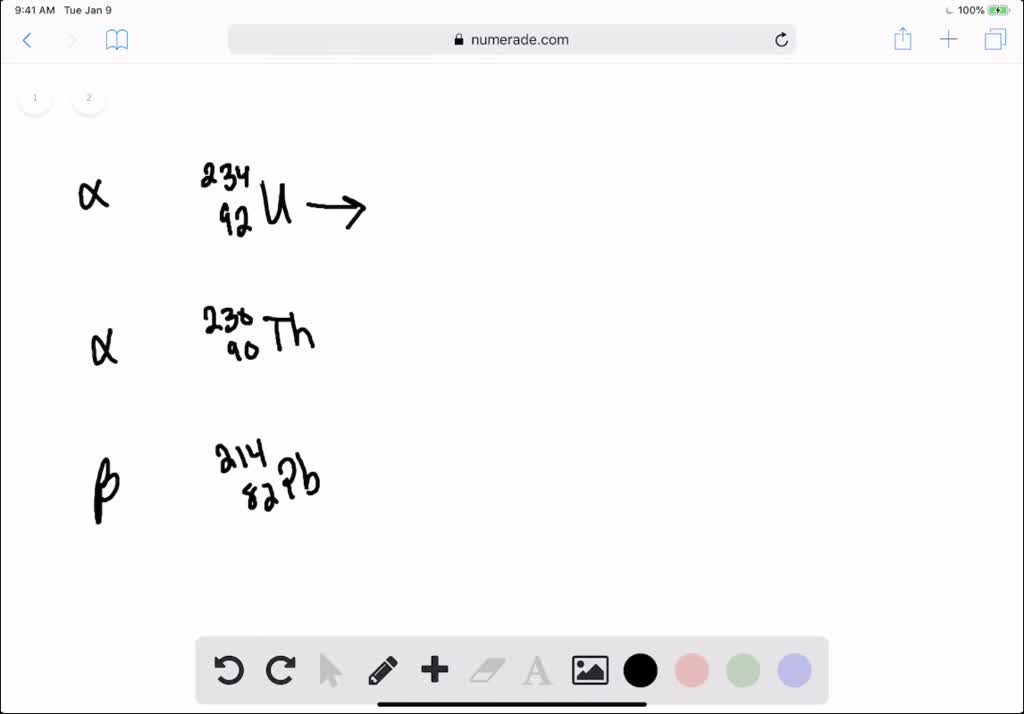 This picture illustrates How to write alpha decay equations.
This picture illustrates How to write alpha decay equations.
How to write balanced nuclear equation for plutonium 244?
A balanced nuclear equation for the alpha decay of plutonium 244 is as follows. The nucleus emits an alpha particle containing two protons and two neutrons. An alpha radioactive decay occurs when the nucleus has too many protons.
How do you write a balanced nuclear equation for Alpha?
Here's how you can do that. When a radioactive nuclide undergoes alpha decay, it emits an alpha particle, 4 2α, which is essentially the nucleus of a helium-4 atom. This means that after the alpha particle is emitted
What happens when an atom emits an alpha particle?
During α-decay, an atomic nucleus emits an alpha particle. It transforms (or decays) into an atom with an atomic number 2 less and a mass number 4 less. Thus, radium-226 decays through α-particle emission to form radon-222 according to the equation:
When does the alpha decay of plutonium occur?
An alpha radioactive decay occurs when the nucleus has too many protons. Plutonium 244 undergoes an alpha radioactive decay to form U- 240 isotope, A balanced nuclear equation for the alpha decay of plutonium 244 is as follows.
Last Update: Oct 2021